Bam 2024 Sf6 Hybridization
Bam 2024 Sf6 Hybridization. This win means that rof has won bam for two years in a row. Understand the molecular shape and hybridization of sf6.
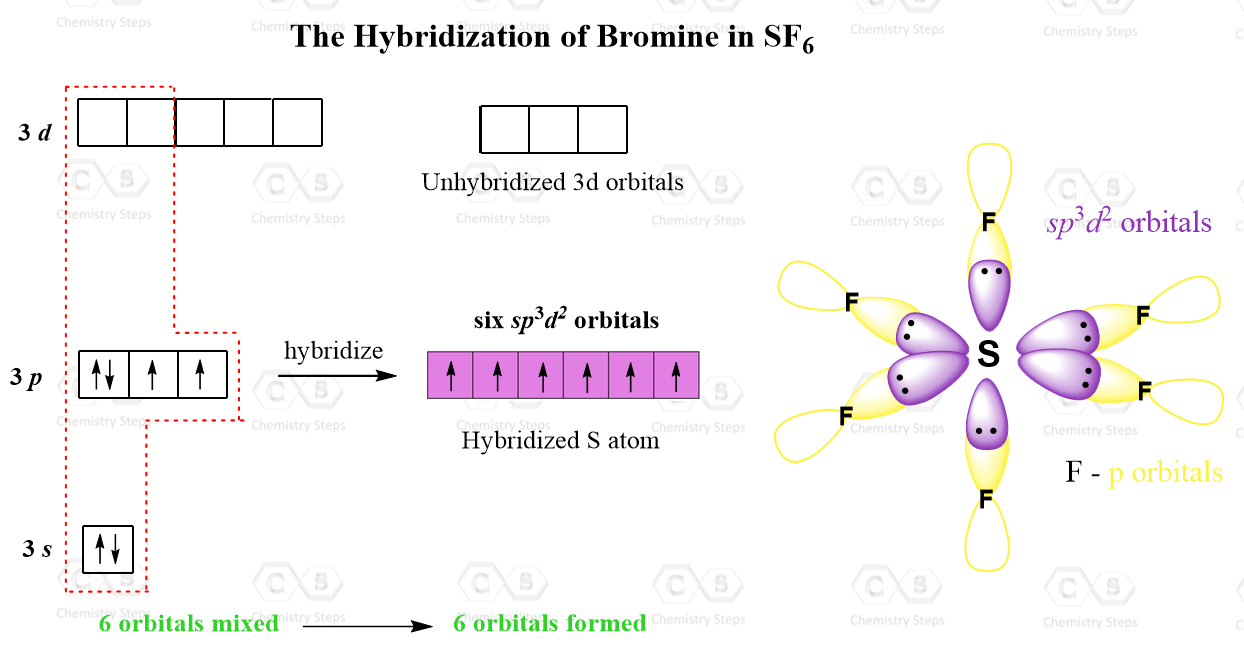
How states can help reduce emissions of sulfur hexafluoride (sf6), a greenhouse gas 23,000 times more potent than carbon dioxide. The resulting \(sp^{3}d^{2}\) hybrid orbitals arrange themselves in an octahedral shape, providing the molecular geometry for \(\text{sf}_6\).
Bam 2024 Sf6 Hybridization Images References :
 Source: topblogtenz.com
Source: topblogtenz.com
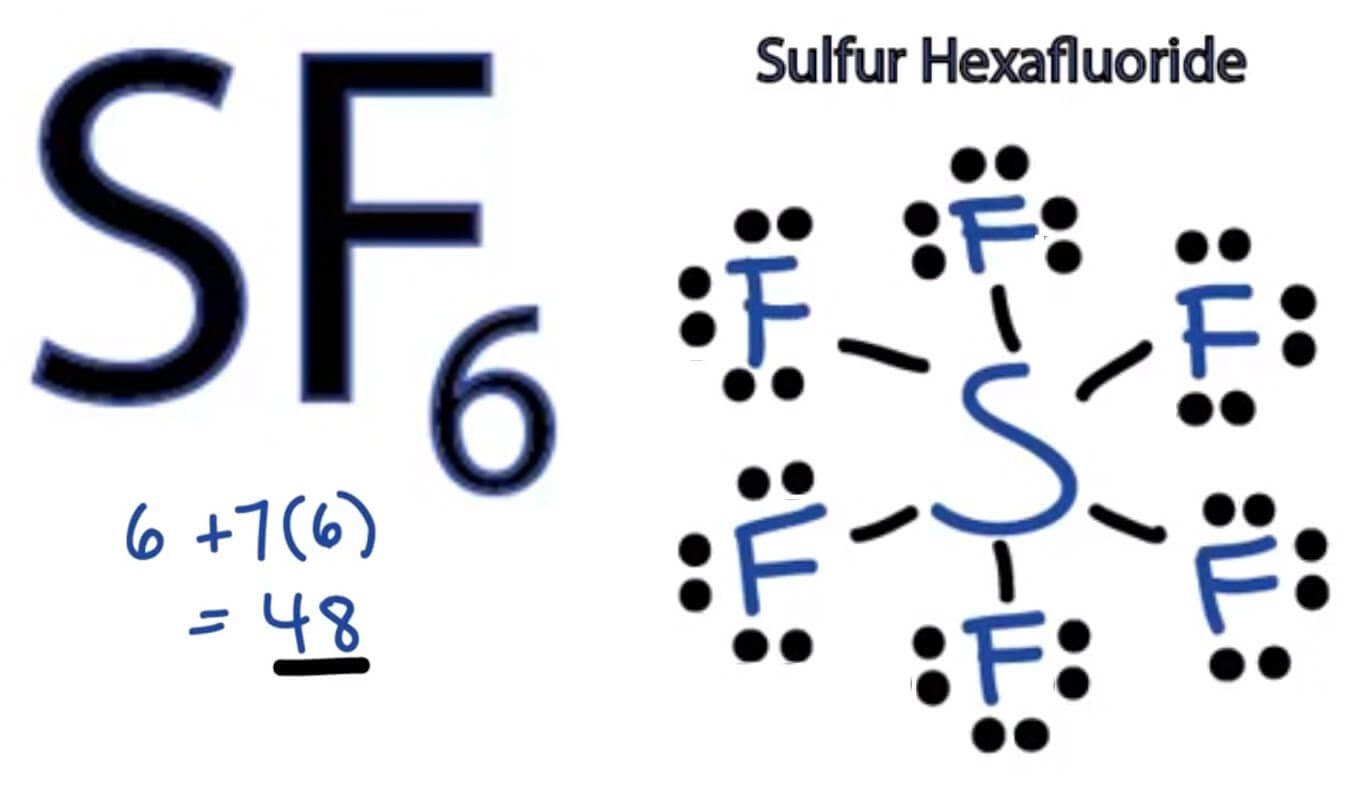
SF6 Lewis structure, Molecular geometry, Bond angle, hybridization, Understand the molecular shape and hybridization of sf6.
 Source: www.youtube.com
Source: www.youtube.com
Hybridization of SF6 (Sulfur Hexafluoride) YouTube, The correct order of hybridization of the central atoms in the following species x e f 2, x e f + 3, x e f 4, x e f 6
 Source: companyprideplatform.org
Source: companyprideplatform.org
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity Company, Here's a recent and arguably more understandable reference:
 Source: techiescientist.com
Source: techiescientist.com
SF6 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram, This is all about the lewis structure of sf6, now let us look at the hybridization and molecular geometry of the compound.
atomic orbitals involved in hybridisation SF6 molecule i, Lewis structure is a representation of bond formation.
 Source: www.youtube.com
Source: www.youtube.com
Sp3d2 hybridization in hindi,Sf6 hybridization,BSC first year, The resulting shape is an octahedron with.
 Source: lacyyodella.pages.dev
Source: lacyyodella.pages.dev
Bam 2024 Sf6 Hybridization Gerty Juliann, This hybridization involves one s orbital, three p orbitals, and two d orbitals from the sulfur atom.
 Source: www.reddit.com
Source: www.reddit.com
BaM January 2024 BuildaMinifig parts (from fateful) r/Legoleak, Here's a recent and arguably more understandable reference:
 Source: lacyyodella.pages.dev
Source: lacyyodella.pages.dev
Bam 2024 Sf6 Hybridization Gerty Juliann, This is all about the lewis structure of sf6, now let us look at the hybridization and molecular geometry of the compound.
 Source: lacyyodella.pages.dev
Source: lacyyodella.pages.dev
Bam 2024 Sf6 Hybridization Gerty Juliann, Axial and equatorial bonds in p cl5 molecule.